Take-All Root Rot: A Complex Disease
go.ncsu.edu/readext?639666
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲Written by Cameron Stephens, a Ph.D. student co-advised by Dr. Jim Kerns and Dr. Travis Gannon
Take-all root rot (TARR) is a disease of ultradwarf hybrid bermudagrass maintained at < 0.2” (5mm) for golf course putting greens. While typically observed on ultradwarf bermudagrass putting greens, other warm-season grasses are susceptible as well. Historically, Gaeumannomyces graminis was considered the causal agent of this disease. However, recent research has associated multiple closely related organisms with take-all root rot (Martin, 2017; Vines et al. 2015). Stand symptoms of take-all root rot include irregular, off-color to white patches. Turfgrass roots appear blackened and necrotic. Darkly pigmented ectotrophic root infecting (ERI) fungal hyphae and hyphopodia (attachment and penetration structures) are ubiquitous on infected root, rhizomes, and stolons. The optimal growing temperatures of TARR organisms ranges between 77-86°F (25-30°C). Disease severity may progress under high heat and increased stress to produce general thinning of non-distinct patches. Older leaves appear chlorotic and in severe cases necrosis at the plant crown is observed. Take-all root rot may also mimic nematode or Pythium root rot injury.
Recently, multiple organisms have been associated with take-all root rot. This has presented a challenge and opportunity for turfgrass pathologists to study the biology of the multiple pathogens associated with this disease. To date, a total of five different organisms across three genera have routinely been isolated from take-all root rot symptomatic turfgrass samples. These organisms are Gaeumannomyces graminis (Gg), Gaeumannomyces sp. (Gx), Gaeumannomyces graminicola (Ggram), Candidacolonium cynodontis (Cc), and Magnaportheopsis cynodontis (Mc). In order to better understand these species and their role in TARR development we evaluated them in pathogenicity and in vitro fungicide sensitivity assays. We also wanted to determine if there was a spatial or seasonal relationship between their distribution and isolation frequency.
Pathogenicity Assay Summary
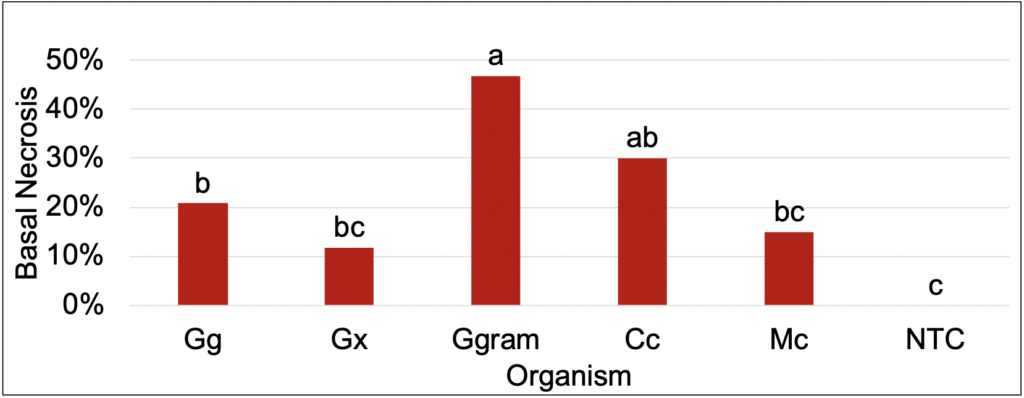
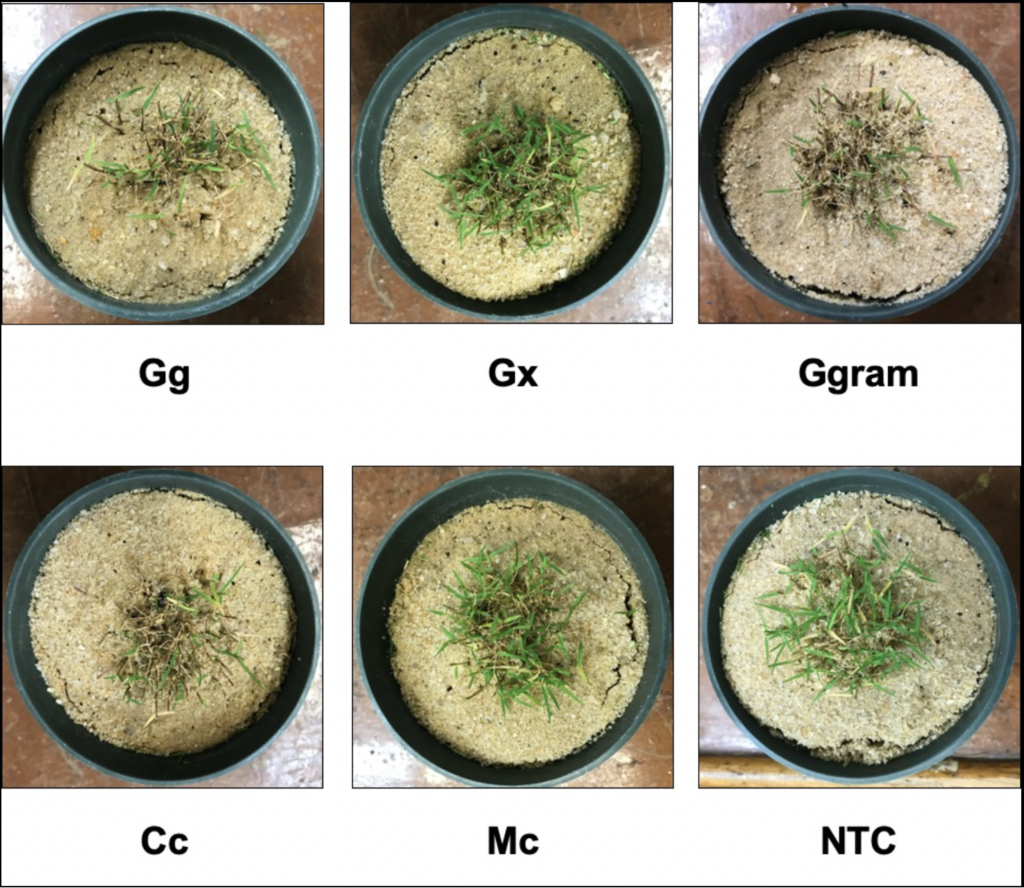
Pathogenicity assays revealed Ggram and Cc produced the most basal necrosis and resulted in the worst turfgrass quality when compared to the non-inoculated control (NTC). However, plants inoculated with Gg also resulted in an increase in basal necrosis and decrease in turfgrass quality. An increase in basal necrosis was negatively correlated with turfgrass quality. Differences in turfgrass quality and basal necrosis can be observed in the figures below.
In vitro Fungicide Sensitivity Assay Summary
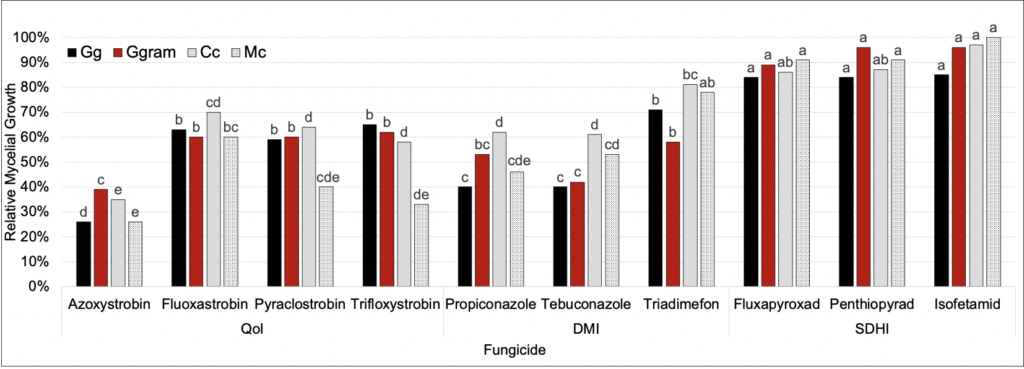
Gg, Ggram, Cc, and Mc were evaluated against 10 different fungicides across 3 different chemical families. In general, the QoI’s and DMI’s provided the most fungal suppression—particularly azoxystrobin, propiconazole, and tebuconazole. Fungicides in the SDHI chemical family did not suppress fungal growth.
TARR Organism Isolation Summary
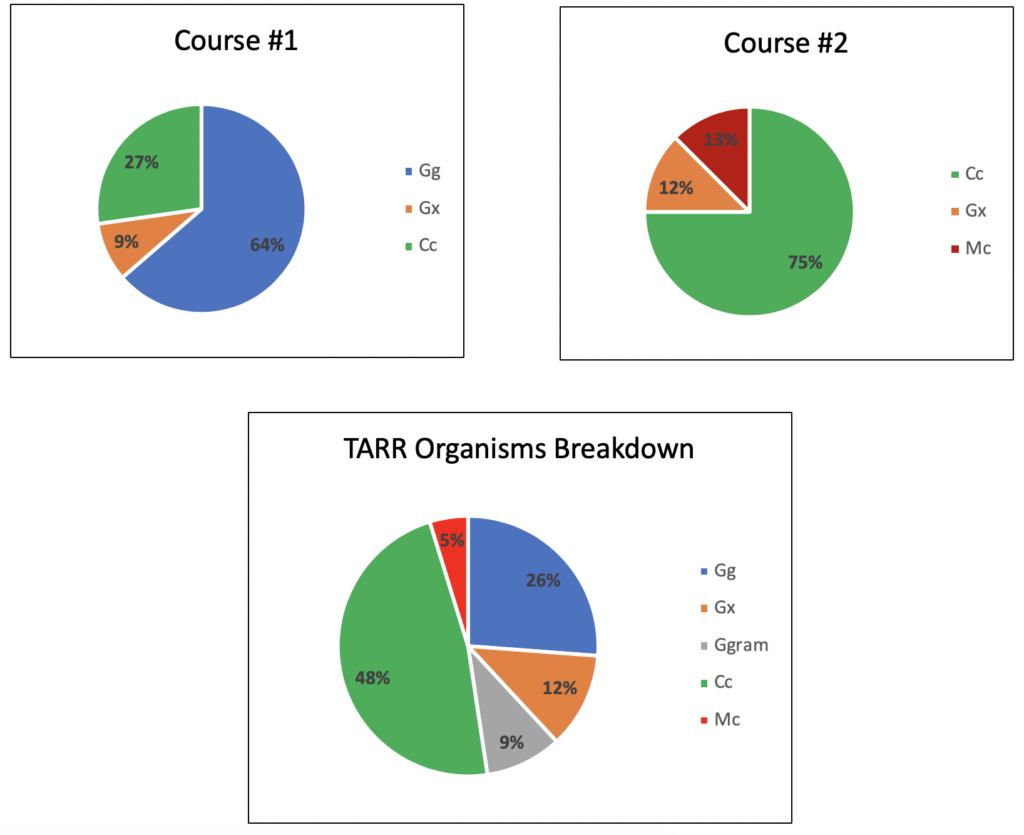
We have currently isolated 42 individual Gg, Ggram, Cc, and Mc isolates from symptomatic roots and stolons and have an additional 40 candidate isolates to identify. From our results we have not noticed any particular geographic or seasonal change in isolation frequency. However, we have noticed that the isolates we are able to obtain from an individual location (i.e. golf course) tend to be a single species. While different TARR organisms responded similarly when evaluated against individual fungicides, certain fungicides provided more suppression of specific organisms. For example, if you primarily have Gg at your golf course, you may get better control when spraying propiconazole when compared to triadimefon. We believe increasing our sample size will continue to shed more light on the population dynamics of TARR and help inform efficacious fungicide practices.
Closing Thoughts
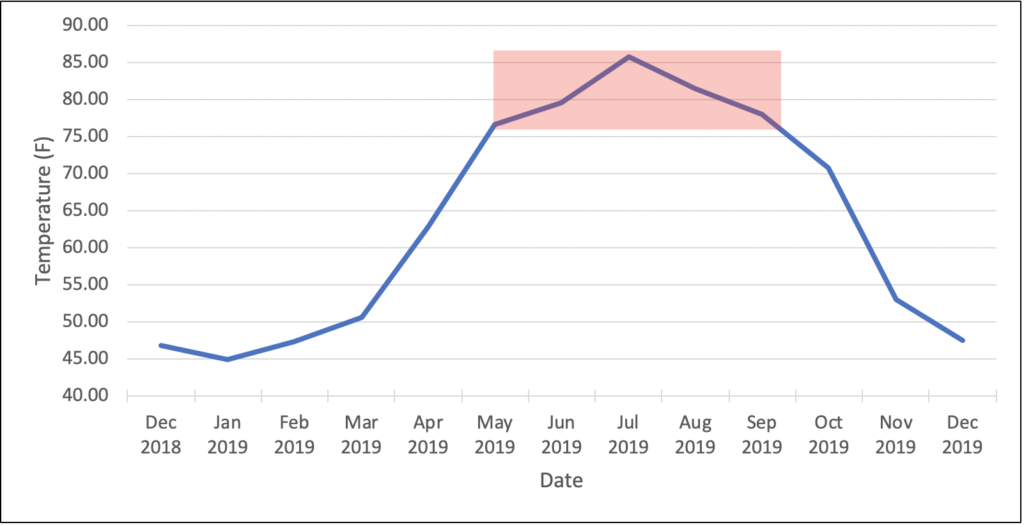
As mentioned above, the optimal growth temperatures of TARR organisms range between 77-86°F (25-30°C). The graph below represents average monthly soil temperature data at our Lake Wheeler Research Station in Raleigh, NC. The red box signifies the months at which average soil temperature is optimal for TARR organism growth—this occurs during May to September in Raleigh. During this time the fungi are most actively growing and potentially doing the most damage to the turf. Fungicides applied during these months may suppress fungal growth the most, therefore this may be optimal timing for preventative applications. We believe the damage done during the summer months begin to manifest in the shoulder seasons once conditions become unconducive for bermudagrass growth (i.e. shorter day length, colder temperatures). We continue to research this complex disease of warm-season turfgrasses and will share new findings as they come available.